Abstract

Background: Blinatumomab, a BiTE® (bispecific T cell engager) immuno-oncology therapy, which redirects CD3+ T cells to engage and lyse CD19+ target cells, has demonstrated efficacy and a tolerable safety profile in patients (pts) with R/R B-ALL when administered as a 28-day continuous intravenous infusion (cIV). SC blinatumomab can simplify treatment administration, improve pt convenience, and reduce cost. Here, we present the dose-escalation results of a multicenter, single-arm, open-label, phase 1b study (NCT04521231) to assess the safety, pharmacokinetics (PK), pharmacodynamics (PD), and efficacy of SC blinatumomab in adults with R/R B-ALL.
Methods: In this ongoing trial, 4 cohorts of up to 6 pts each were planned. Pts in each cohort received 2‒5 cycles of SC blinatumomab. Each cycle included a 26-day treatment period and a 1-week treatment-free interval. Pts in cohorts 1, 2, 3, and 4 received 40, 120, 250, or 500 µg of SC blinatumomab once daily, respectively, from days 1 to 7. This was followed by a thrice-weekly regimen of 250 µg SC blinatumomab for cohorts 1 and 2 and 500 and 1000 µg of SC blinatumomab for cohorts 3 and 4, respectively, from days 8 to 26 of cycle 1, and from days 1 to 26 for all subsequent cycles. Bone marrow (BM) evaluation was performed on day 27 of each cycle and additionally on day 12 of cycle 1 in cohorts 3 and 4.
Results: Twenty pts were enrolled at the data cut-off of June 20, 2022 (6 in cohort 1, 3 in cohort 2, 5 in cohort 3, and 6 in cohort 4). Median age was 58 years (range, 19‒83). Ten pts (50%) were aged >55 years. The number of prior therapies ranged from 2 to 4. All pts had an Eastern Cooperative Oncology Group score of 0-1 at enrollment. Six pts were refractory to frontline or salvage therapy, 9 pts relapsed after chemotherapy, 3 pts relapsed after prior hematopoietic stem cell transplantation (HSCT), 1 pt relapsed after prior HSCT and anti-CD19 chimeric antigen receptor T cell therapy, and 1 pt relapsed after 2 prior HSCTs and blinatumomab therapy where initial residual disease was cleared with cIV blinatumomab enabling first HSCT. The median number of cycles of SC blinatumomab received was 1 (range, 1‒5). Seven pts received 1 cycle, 1 pt received 2, 1 pt received 4 and 2 pts received 5. Six pts ended treatment during cycle 1 and in 3 pts cycle 1 is ongoing. Median bone marrow blast count at the start of the study was 77% (range, 6‒100%).
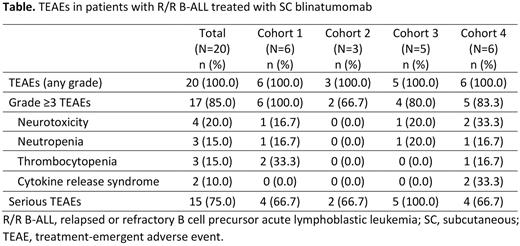
No dose-limiting toxicities were reported in any cohort. Seventeen pts had grade ≥3 treatment-emergent adverse events (TEAEs; Table). Six pts (30.0%) had neurotoxicity (NT) at any grade and 4 pts (20%) had NT at grade 3. Sixteen pts (80.0%) had cytokine release syndrome (CRS) at any grade and 2 pts (10.0%) had CRS at grade 3 (cohort 4; each event resolved within 48 h and subsequent cycle 1 dose was restarted). There was no incidence of NT or CRS at grade ≥4. Six pts ended treatment due to TEAEs, of which 4 pts were from cohort 1 (n=1, grade 5 herpes encephalitis unrelated to blinatumomab; n=1, injection site reaction in cycle 2 in pt with no response; n=1, hyperleukocytosis due to disease progression; n=1, face swelling due to progression of extramedullary disease) and 2 pts were from cohort 3 (n=1, grade 2 CRS, grade 3 liver enzyme elevation, and grade 3 NT [dysarthria and disorientation]; n=1, disease non-response).
Preliminary PK results through cohort 3 (cohort 4 ongoing) showed that observed exposures (average concentrations at steady state [SS]) of SC blinatumomab were consistent with the efficacious exposures (SS concentration) of the approved regimen of cIV blinatumomab. The PD profile demonstrated that peripheral T cell redistribution and activation (CD3+ and CD8+ CD69+ T cells), transient cytokine elevation (IL-6, IL-10, IFN-γ), and CD19+ B cell depletion were consistent with the historical PD profile for cIV blinatumomab.
Nine of 14 pts (64.3%) in cohorts 1‒3 (cohort 4 ongoing; 3 of 6 [50%], 2 of 3 [66%], 4 of 5 [80%] in cohorts 1, 2, and 3, respectively) achieved complete response with full or partial hematologic recovery within 2 cycles of SC blinatumomab without the presence of measurable residual disease (MRD<10-4).
Conclusion: In this ongoing phase 1b dose-escalation study, SC blinatumomab demonstrated an acceptable safety profile and anti-leukemia activity in heavily pretreated pts with R/R B-ALL. PK exposures and PD profiles were consistent with those reported for the cIV regimen of blinatumomab, supporting the use of SC dosing of blinatumomab in this pt population.
Disclosures
Zugmaier:Micromet/Amgen: Patents & Royalties: Patents 20190300609 and 20130323247 licensed; Receives royalties of family members of international applications published as WO2010/052014; WO2010/052013; WO2011/051307; WO2012/055961; WO2012/062596; WO2014/122251; and WO2015/181683; Amgen: Current Employment, Current equity holder in publicly-traded company. Jabbour:Spectrum: Research Funding; Pfizer: Other: Advisory Role, Research Funding; Amgen: Other: Advisory Role, Research Funding; Genentech: Other: Advisory Role, Research Funding; Bristol Myers Squibb: Other: Advisory Role, Research Funding; Takeda: Other: Advisory Role, Research Funding; Adaptive Biotechnologies: Other: Advisory Role, Research Funding; AbbVie: Other: Advisory Role, Research Funding. Schwartz:Amgen: Honoraria, Other: Advisory Board; CSI GmbH (Amgen/Jazz Pharmaceuticals): Speakers Bureau. Huguet:novartis: Honoraria; incyte: Honoraria; bms: Honoraria; amgen: Honoraria; pfizer: Honoraria; jazz pharma: Honoraria. Hernández-Rivas:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GSK: Consultancy, Honoraria; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research Support, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research support, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Research Support; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees; Lilly: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Rovi: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees. Lussana:Amgen: Honoraria; Astellas Pharma: Honoraria; Pfizer: Honoraria; Incyte: Honoraria; Janssen Oncology: Honoraria; AbbVie: Consultancy. Wong:Amgen: Current Employment. Rambaldi:Celgene-BMS: Honoraria; Janssen: Honoraria; Roche: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Kite-Gilead: Honoraria; Jazz: Honoraria; ABBVIE: Honoraria; Astellas: Honoraria; Pfizer: Honoraria; Amgen: Honoraria; Omeros: Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal